Given :
Mass of given magnesium chloride, m = 256 g.
To Find :
How many grams of chloride are there in 256 g of magnesium chloride.
Solution :
Molecular formula of magnesium chloride is
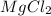 .
.
Molecular formula of
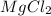 is, M = 95.211 g/mol .
is, M = 95.211 g/mol .
Mass of chlorine in 1 mol of
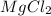 is , m = 35.5 × 2 = 71 g.
is , m = 35.5 × 2 = 71 g.
So, amount of chlorine in 256 gram
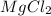 :
:
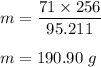
Hence, this is the required solution.