Answer:
The mass of silver that will occupy a volume of 87.75 mL is 920.5 grams.
Step-by-step explanation:
Density is defined as the property that matter, whether solid, liquid or gas, has to compress into a given space. In other words, density is a quantity that allows us to measure the amount of mass in a certain volume of a substance. Then, the expression for the calculation of density is the quotient between the mass of a body and the volume it occupies:
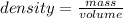
So the density of silver is 10.49 g / mL indicates that 10.49 g occupies a volume of 1 mL. Then a rule of three can be applied in the following way: if in 1 mL a mass of 10.49 g is occupied, in 87.75 mL how much mass will it occupy?
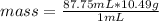
mass= 920.5 g
The mass of silver that will occupy a volume of 87.75 mL is 920.5 grams.