Answer:
Protons: 2.
Electrons: 2.
Neutrons: 2.
Step-by-step explanation:
Hello,
In this case, since an atom's atomic number is equal to the number of electrons, considering the electron configurations, taking into account that helium-4 is neither positively nor negatively charged, we can infer that the number of electrons equal the number of protons, which in this case are 2, due to the fact that is atomic number is 2.
Moreover, as helium-4's atomic number is 4 as a whole number, we compute the number of neutrons by using the shown below equation:
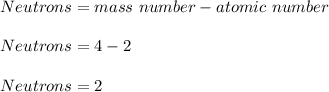
Regards.