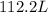Answer:
112.2L
Step-by-step explanation:
Volume (V) = 300g
Temperature (T) = 822K
Pressure (P) = 0.9atm
using the ideal gas equation;
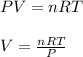
Molar gas constant (R) =
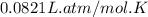
Mole (n) =
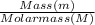 Molar mass of Mercury = 200.59g/mol
Molar mass of Mercury = 200.59g/mol
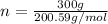
= 1.496mol
Now, the volume can be calculated;
V =
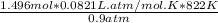
∴Volume of mercury =