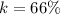Answer:
The value is
Step-by-step explanation:
From the question we are told that
The mass of toluene
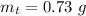
The mass of potassium permanganate is
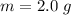
The volume of potassium hydroxide V = 7.0 mL
The concentration of potassium hydroxide C = 6 M
The mass of benzoic acid is
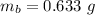
Generally the % yield of benzoic acid is mathematically represented as
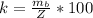
Here Z is the theoretical yield which is mathematically represented as

Here W is the molecular weight of product (benzoic acid) with value
W = 92.14 \ g
E is the molecular weight of reactant (toluene)with a constant value of
E = 122.12 g
So
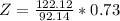
=>
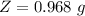
So
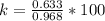
=>