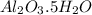Answer: The formula of the hydrated alumina is
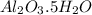
Step-by-step explanation:
Decomposition of hydrated alumina is given by:
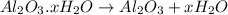
Molar mass of
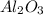 = 101.96 g/mol
= 101.96 g/mol
According to stoichiometry:
(101.96+18x) g of
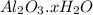 decomposes to give 101.96 g of
decomposes to give 101.96 g of
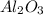
Thus 5.000 g of
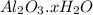 decomposes to give=
decomposes to give=
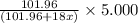 of H_2O
of H_2O
But it is given 5.000 g of a sample of hydrated salt
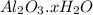 was found to contain 2.6763 g of unhydrated salt
was found to contain 2.6763 g of unhydrated salt
Thus we can equate the two equations:
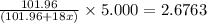
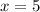
Thus the formula of the hydrated alumina is