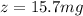Answer:
The value is
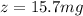
Step-by-step explanation:
From the question we are told that
The mass of 9-fluorenone is m = 19 mg
The volume of diethyl ether V = 5 ml
The volume of water is
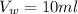
The equilibrium constant for 9-fluorenone is
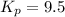
Generally the equilibrium constant for 9-fluorenone is mathematically represented as
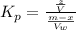
Here z is the remaining mass of 9-fluorenone
=>
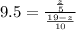
=>
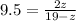
=>