Answer:
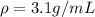
Step-by-step explanation:
Hello,
In this case, considering that the density of a body is computed given its mass and volume:
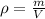
Taking into account that the mass is 24.32 g and the volume is computed via the difference between the volume of the water with the brass and the volume of water by itself as follows:
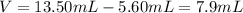
The density of the piece of brass turns out:
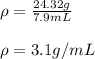
Best regards.