Answer:
Double displacement or metathesis reaction.
Step-by-step explanation:
Hello,
In this case, the correct reaction is:
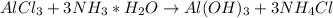
Which is a double displacement or metathesis reaction since the hydrated ammonia is now bonded with the chloride ions which where also substituted by hydroxyl ions on aluminium. In this case, we verify that aluminum chloride reacts with ammonia-water in order to form aluminium hydroxide and ammonium chloride.
Regards.