Given :
Heat absorbs , Q = 10 J .
Pressure ,
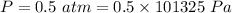
Initial volume ,
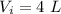 .
.
Final volume ,
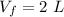 .
.
To Find :
The change in the internal energy .
Solution :
By first law of thermodynamics :
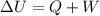
Here , W is work done ,
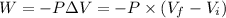
Therefore,
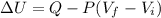
Putting values in SI units in above equation :
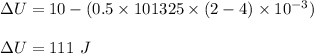
Therefore , option b. is correct .
Hence , this is the required solution .