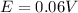Complete Question
A voltaic cell is constructed with two
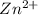
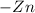 electrodes. The two cell compartment have
electrodes. The two cell compartment have
![[Zn^(2+)] = 1.6 \ M](https://img.qammunity.org/2021/formulas/physics/college/xtzrbf5cyvjikj71nzwn1r70ww3ikl6n1e.png) and
and
![[Zn^(2+)] = 2.00*10^(-2) \ M](https://img.qammunity.org/2021/formulas/physics/college/nuvqx5zo2xr60ikdb5ynd068g36unyezoa.png) respectively.
respectively.
What is the cell emf for the concentrations given? Express your answer using two significant figures
Answer:
The value is
Step-by-step explanation:
Generally from the question we are told that
The concentration of
![[Zn^(2+)]](https://img.qammunity.org/2021/formulas/physics/college/3s1xv5vmch57ma9170iwp5lkdr1r55cqap.png) at the cathode is
at the cathode is
![[Zn^(2+)]_a = 1.6 \ M](https://img.qammunity.org/2021/formulas/physics/college/m72pbvbey4q62jk1393wlez1q31npmjqy6.png)
The concentration of
![[Zn^(2+)]](https://img.qammunity.org/2021/formulas/physics/college/3s1xv5vmch57ma9170iwp5lkdr1r55cqap.png) at the anode is
at the anode is
![[Zn^(2+)]_c = 2.00*10^(-2) \ M](https://img.qammunity.org/2021/formulas/physics/college/fgml9tfldfqrgn5qirtteg8o63x3hum35k.png)
Generally the the cell emf for the concentration is mathematically represented as
![E = E^o - (0.0591)/(2) log([Zn^(2+)]a)/( [Zn^(2+)]c)](https://img.qammunity.org/2021/formulas/physics/college/lxtyhgv725qii71y7psk7g9difyiv8c4u4.png)
Generally the
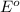 is the standard emf of a cell, the value is 0 V
is the standard emf of a cell, the value is 0 V
So
![E = 0 - (0.0591)/(2) * log[( 2.00*10^(-2))/(1.6) ]](https://img.qammunity.org/2021/formulas/physics/college/1bks9mwst6oy7jijfxy94ebnarb6xhgi9n.png)
=>