Answer:
c: is a good conductor of electricity
Step-by-step explanation:
One of the physical properties of hydrochloric acid is that it is a good conductor of electricity in an aqueous state.
The dissolution of hydrochloric acid in water causes a complete dissociation into
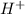 and
and
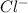 . The free-floating ions that result from the dissolution enable hydrochloric acid to be able to conduct electricity.
. The free-floating ions that result from the dissolution enable hydrochloric acid to be able to conduct electricity.
In aqueous solution, the attraction of the positive dipole of the hydrochloric acid to the negative dipole of water and that of the positive dipole of water to the negative dipole of the hydrochloric acid creates a dipole-dipole interaction that allows complete dissociation of the hydrochloric acid. Thus, electrolyte results.
Correct option: c