Answer:
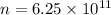
Step-by-step explanation:
We need to find the number of electrons that would have to be removed from a coin to leave it with a charge of
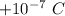 . Then the number of electrons be n. Using quantization of electric charge as :
. Then the number of electrons be n. Using quantization of electric charge as :
q = ne
e is charge on an electron
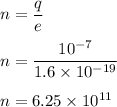
So, the number of electrons are
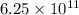 .
.