Answer:
0.14%
Step-by-step explanation:
The computation of % is shown below:
As we know that
HClO <=> H+ + ClO-
I 0.015 0 0
C -a +a +a
E 0.015-a a a
Now
![Ka = ([H+][ClO-])/([HClO])](https://img.qammunity.org/2021/formulas/chemistry/college/6agqsdkr8g9a1esppxik3r9r41p84bjeoy.png)
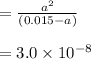
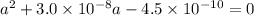
Now Solves the quadratic equation i.e.
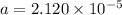
![[H+] = a = 2.120 * 10^(-5) M](https://img.qammunity.org/2021/formulas/chemistry/college/23r2co9av82kvop1nrwjnwf9pyfmyjqw6a.png)
So,
% ionization is
![= ([H+])/([HClO])_(initial) * 100\%\\\\= 2.120 * 10^(-5)/0.015 * 100\%](https://img.qammunity.org/2021/formulas/chemistry/college/m1l93zrs1giz5o528mzj367jz9xfbvzqik.png)
= 0.14%
Hence, the percentage of hypochlorous ionization is 0.14%