Answer:
D. Pure gold
Step-by-step explanation:
Hello,
In this case, since gold, as a heavy metal, is said to be less reactive than hydrogen, when it undergoes electrolysis process when forming a salt, due to the action of the electric current, we can appreciate the formation of a layer of gold on the surface of the cathode via a reduction half-reaction from gold (I) to metallic gold:
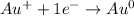
Thereby, D. Pure gold is formed as the product of the reaction.
In contrast, more reactive metals than hydrogen such as sodium or potassium, will remain in solution so the hydrogen converted to hydrogen gas.
Best regards-