Answer:
6.87 g/mL
Explanation:
The density of an object can be found by dividing the mass by the volume.
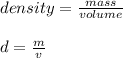
We know that the aluminum occupies a volume of 12.7 milliliters and weighs 87.3 grams. Therefore, the mass is 87.3 g and the volume is 12.7 mL.
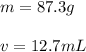
Substitute the values into the formula.
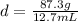
Divide 87.3 g by 12.7 mL
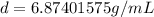
Round to the nearest hundredth. The 4 in the thousandth place tells us to leave the 7 in the hundredth place.
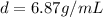
The density of the aluminum is about 6.87 grams per milliliter.