Answer:
A. Disturbing the equilibrium by adding NO2Cl decreases Qc to a value less than Kc.
B. To reach a new state of equilibrium, Qc therefore increases which means that the denominator of the expression for Qc decreases.
C. To accomplish this, the concentration of reagents decreases, and the concentration of products increases.
Step-by-step explanation:
Hello,
In this case, for the equilibrium reaction:
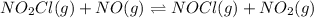
Whose equilibrium expression is:
![Kc=([NO_2][NOCl])/([NO_2Cl][NO])](https://img.qammunity.org/2021/formulas/chemistry/college/hrg99z03r3t3lj0y3tlloc0f1pgrn7jgou.png)
The proper matching is:
A. Disturbing the equilibrium by adding NO2Cl decreases Qc to a value less than Kc, since the denominator becomes greater, therefore, Qc decreases.
B. To reach a new state of equilibrium, Qc therefore increases which means that the denominator of the expression for Qc decreases, since the lower the denominator, the higher Qc as it has the concentration of reactants.
C. To accomplish this, the concentration of reagents decreases, and the concentration of products increases, since the reactants must be consumed in order to reestablish equilibrium by shifting the reaction towards the products.
Best regards.