Answer:
C. CrF6, chromium(VI) hexafluoride.
Step-by-step explanation:
Hello,
In this case, since we are given a hexavalent chromium we must notice it has +6 as its oxidation state. Moreover, fluorine, when forming ionic compounds works with -1, for which the chemical formula is:
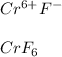
And the stock name is indeed C. CrF6, chromium(VI) hexafluoride (looks like D. is the same) since we have six fluoride ions in the formula and we point out chrmium's oxidation state.
Regards.