Answer:
Osmotic pressure of the solution will be 818.203 Pa.
Step-by-step explanation:
Osmotic pressure of a solution is defined by the formula,
π = MRT
where π = Osmotic pressure
M = Molarity of the solution
R = Ideal gas constant
T = Temperature in °K
40 grams of glucose was dissolved in water so the Molarity of the solution will be,
M =
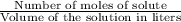
Moles of solute =
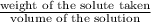
=
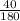
=
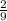 moles
moles
Morarity =
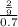
= 0.3175 mole per liter
Value of ideal gas constant 'R' = 8.314
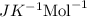
T = 37°C = (273 + 37)° K
= 310°K
Now by substituting these values in the formula,
π =
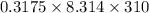
= 818.203 Pa
Therefore, osmotic pressure of the solution will be 818.203 Pa.