Answer:
See figure 1
Step-by-step explanation:
In this case, we have to start with the ionization reaction of HBr to produce the hydronium ion (
 ) and the bromide ion (
) and the bromide ion (
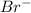 ). Then the double bond in the alkene can attack the hydronium ion to produce a carbocation. The most stable carbocation would be the tertiary one, therefore we have to put the positive charge in the tertiary carbon. Then, the bromide attacks the carbocation to produce the final halide.
). Then the double bond in the alkene can attack the hydronium ion to produce a carbocation. The most stable carbocation would be the tertiary one, therefore we have to put the positive charge in the tertiary carbon. Then, the bromide attacks the carbocation to produce the final halide.
See figure 1
I hope it helps!