Answer:
Sn2 mechanism reaction
Step-by-step explanation:
In this case, we have a primary substrate (1-bromo-3,3-dimethylbutane). Because the leaving group "Br" is bonded to a primary carbon. Additionally, the nucleophile will come from the "NaI" (sodium iodide). This is an ionic compound, so, in solution, a cation and an anion would be produced. The anion
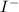 would be the nucleophile.
would be the nucleophile.
Due to the primary substrate, we will have an Sn2 reaction. So, the attack of the nucleophile and the removal of the leaving group will take place in 1 step. Producing a "transition state" and finally and the final product (1-iodo-3,3-dimethylbutane).
See figure 1
I hope it helps!