Answer:
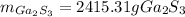
Step-by-step explanation:
Hello,
In this case, for the given chemical reaction, we can notice there is a 4:2 molar ratio between the burned moles of gallium and the yielded moles of gallium sulfide, therefore, we compute them as shown below:
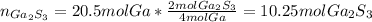
Then, by using the molar mass of gallium sulfide (235.64 g/mol), we directly compute the grams:
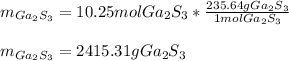
Best regards.