Answer:
7 (neutral).
Step-by-step explanation:
Hello,
In this case, for the chemical reaction:

We can notice that since hydrochloric acid and sodium hydroxide are strong, they will fully dissociate during the titration, therefore, as they are in stoichiometric proportions in equal concentrations for the equivalence point, the pH will be 7 (neutral) since all the chloride ions are neutralized by the sodium ions.
Moreover, for the given acid solution, the required volume of sodium hydroxide will be:
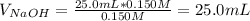
To attain a complete titration until the equivalence point.
Best regards.