Answer:
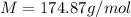
Step-by-step explanation:
Hello,
In this case, we consider the depression in the freezing point as:
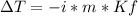
Whereas the van't hoff factor for ascorbic acid is 1 since it is covalent, thus, we solve for the molality as shown below:

Next, since molal units are mol/kg, we can compute the present moles of ascorbic acid in the 250 g (0.25kg) of water:
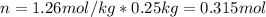
Finally, the molar mass with the given 55.0 g of ascorbic acid:
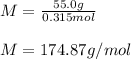
Regards.