Answer:
The correct answer is - 0.570 grams
Step-by-step explanation:
The balanced chemical reaction is given by
Cu(NO3)2(aq) + 2NaOH(aq) --------> Cu(OH)2(s) + 2NaNO3(aq)
1.0 mole 2.0 mole 1.0 mole 2.0 mole
number of mol of Cu(OH)2,
n = Molarity * Volume
=
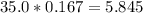 millimoles
millimoles
As clear in the equation, 1 mole of Cu(NO3)2 gives 1 mole of Cu(OH)2 , So, 5.845 millimoles of Cu(NO3)2 will produce 5.845 millimoles of Cu(OH)2
Mass of Cu(OH)2 = number of mol * molar mass
=
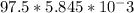
= 0.570 grams
Thus, the correct answer is - 0.570 grams