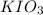Complete Question
The complete question is shown on the first uploaded image
Answer:
The initial concentration is
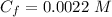
Step-by-step explanation:
From the question we are told that
The volume of solution A is
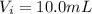
The concentration of A is
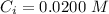
The volume of solution B is
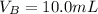
The volume of water is
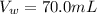
Generally the law of dilution is mathematically represented as
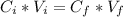
Where
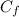 is the concentration of the mixture
is the concentration of the mixture
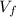 is the volume of the mixture which is mathematically evaluated as
is the volume of the mixture which is mathematically evaluated as
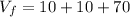
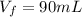
So
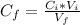
substituting values
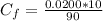
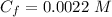
Note the mixture obtained is