Answer:
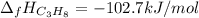
Step-by-step explanation:
Hello,
In this case, we can consider that the given heat of combustion is indeed the heat of reaction since it corresponds to the combustion of propane, which is computed by using the heat formation of all the involved species as shown below:
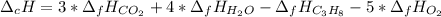
Thus, since the heat of formation of gaseous carbon dioxide is -393.5 kJ/mol, water -241.8 kJ/mol and oxygen 0 kJ/mol, the heat of formation of propane is:
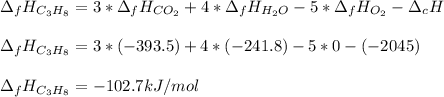
Best regards.