Answer:
The fraction of the cooper's electrons that is removed is
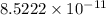 .
.
Step-by-step explanation:
An electron has a mass of
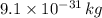 and a charge of
and a charge of
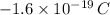 . Based on the Principle of Charge Conservation,
. Based on the Principle of Charge Conservation,
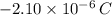 in electrons must be removed in order to create a positive net charge. The amount of removed electrons is found after dividing remove charge by the charge of a electron:
in electrons must be removed in order to create a positive net charge. The amount of removed electrons is found after dividing remove charge by the charge of a electron:
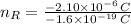
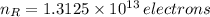
The number of atoms in 56 gram cooper ball is determined by the Avogadro's Law:
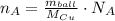
Where:
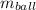 - Mass of the ball, measured in kilograms.
- Mass of the ball, measured in kilograms.
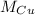 - Atomic mass of cooper, measured in grams per mole.
- Atomic mass of cooper, measured in grams per mole.
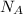 - Avogradro's Number, measured in atoms per mole.
- Avogradro's Number, measured in atoms per mole.
If
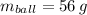 ,
,
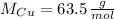 and
and
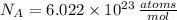 , the number of atoms is:
, the number of atoms is:
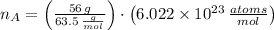
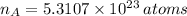
As there are 29 protons per each atom of cooper, there are 29 electrons per atom. Hence, the number of electrons in cooper is:
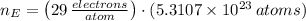
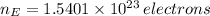
The fraction of the cooper's electrons that is removed is the ratio of removed electrons to total amount of electrons when net charge is zero:
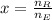
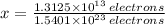
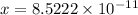
The fraction of the cooper's electrons that is removed is
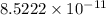 .
.