Answer:
correct option: 2 -> It would decrease.
Explanation:
If the amount of gas is the same, the following relation needs to be constant:
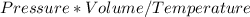
So, If the pressure is constant, the volume and the temperature are directly proportional (if one increases, the other also increases).
Then, an decrease of 3 times in the volume would cause an decrease of 3 times in the temperature.
So the correct option is 2.: It would decrease.