Answer : The expression for acid dissociation constant will be:
![K_a=([H^+][H_2BO_3^-])/([H_3BO_3])](https://img.qammunity.org/2021/formulas/chemistry/high-school/qr3giuvxm34enzd7k4mw350e4mcv0l049g.png)
Explanation :
Acid dissociation constant : It is an equilibrium constant that refers to the dissociation or ionization of an acid.
It is denoted as
 .
.
The given equilibrium reaction is:
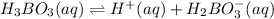
The expression for acid dissociation constant will be:
![K_a=([H^+][H_2BO_3^-])/([H_3BO_3])](https://img.qammunity.org/2021/formulas/chemistry/high-school/qr3giuvxm34enzd7k4mw350e4mcv0l049g.png)