Answer:
b. 760 g
Step-by-step explanation:
The mass of the solution = 800 g
5% of NaCl by mass of the solution can be determined as follows;
5% of 800 =
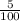 × 800
× 800
= 5 × 8
= 40 g
The mass of NaCl in the solution is 40 g.
The mass of water = mass of solution - mass of NaCl
= 800 - 40
= 760 g
Therefore, the mass of water required is 760 g.