Answer:
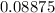
Step-by-step explanation:
Hello,
In this case, the first step is to compute the difference in the electronegativity for the formed bond between gallium and phosphorous by:
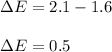
Thus, we can compute the percentage of ionic character by:
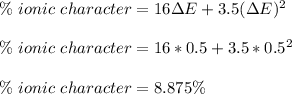
So the fraction is just:
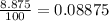
Which has sense since gallium phosphide is a non-polar compound.
Regards.