Answer:
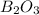
Step-by-step explanation:
First, we have to find the reaction:
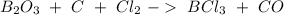
The next step is to balance the reaction:
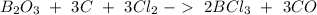
Now, we have to calculate the molar mass for each compound, so:
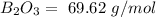
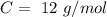
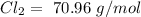
With these values, we can calculate the moles of each compound:
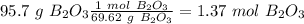
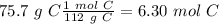
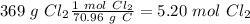
Now we can divide by the coefficient of each compound in the balanced equation:
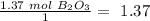
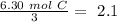
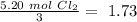
The smallest values are for
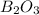 , so this is our limiting reagent.
, so this is our limiting reagent.
I hope it heps!