Answer: 0.225 atm
Step-by-step explanation:
For this problem, we have to use Boyle's Law.
Boyle's Law: P₁V₁=P₂V₂
Since we are asked to find P₂, let's manipulate the equation.
P₂=(P₁V₁)/V₂
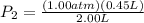
With this equation, the liters cancel out and we will be left with atm.
P₂=0.225 atm