Answer:
The initial temperature will be "385.1°K" as well as final will be "128.3°K".
Step-by-step explanation:
The given values are:
Helium's initial volume, v₁ = 6 m³
Mass, m = 1.5 kg
Final volume, v₂ = 2 m³
Pressure, P = 200 kPa
As we know,
Work,
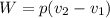
On putting the estimated values, we get
⇒
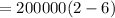
⇒
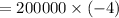
⇒
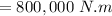
Now,
Gas ideal equation will be:
⇒
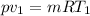
On putting the values. we get
⇒
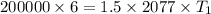
⇒
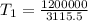
⇒
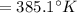 (Initial temperature of helium)
(Initial temperature of helium)
and,
⇒
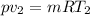
On putting the values, we get
⇒
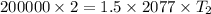
⇒
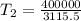
⇒
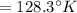 (Final temperature of helium)
(Final temperature of helium)