Answer:
Final pressure =
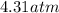
Step-by-step explanation:
According to Gay-Lussac's law the pressure of a given mass of gas varies directly with the absolute temperature of the gas, provided the volume is kept constant.
SEE THE ATTACHMENT BELOW FOR STEP BY STEP EXPLANATION