Answer:
0.25 mol
Step-by-step explanation:
Use the formula n=N/NA
n= number of mols
N = number of particles
Nᵃ = Avogadros constant = 6.02 x
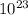
So, n=
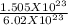
The 10 to the power of 23 cancels out and you are left with 1.505/6.02, which is approximately 1/4. This is the same as 0.25 mol.
Hope this helped :)