Answer:
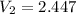 atm
atm
Step-by-step explanation:
As we know that
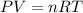
Where P is the pressure in atmospheric pressure
T is the temperature in Kelvin
R is the gas constant
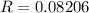
V is the volume in liters
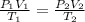
Substituting the given values in above equation, we get -
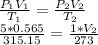
On rearranging, we get
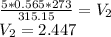
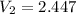 atm
atm