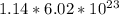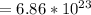Answer:
Molecules of oxygen
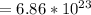
Step-by-step explanation:
The balanced equation of reaction between iron and oxygen is
4 Fe + 3 O2 → 2 Fe2O3
4 moles of iron react with 3 moles of oxygen.
Mass of one mole of iron is 55.845
number of iron moles in 84.9 grams of solid iron is equal to
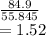
Now 1 mole of iron will react with
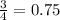 mole of oxygen
mole of oxygen
Thus 1.52 moles of iron will react with
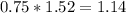 moles of oxygen
moles of oxygen
Number of atoms of oxygen in 1.14 moles