Answer:
Heat, Q = 45 J
Step-by-step explanation:
We have,
Mass of aluminium is 5 g
The temperature is raised from 22°C to 32°C.
The specific heat of aluminium is 0.90 J/g°C.
It is required to find the heat are needed to raise the temperature.
The heat required to raise the temperature is given by :
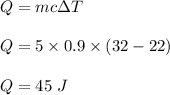
So, 45 J of heat are needed to the temperature of 5.0 g of aluminum from 22°C to 32°C.