Answer:
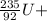
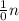 ------->
------->
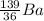 +
+
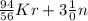

Step-by-step explanation:
In equation 1, equating the mass number (A) on both sides.
A = 235 + 1 = A + 94 + 3*1
236 = A + 94 + 3
A = 236 - 94 = 3
A = 139
Equate the atomic numbers on both sides
92 + 0 = Z + 36 + 3*0
92 = Z + 36
Z = 92 - 36
Z = 56
In reaction 2, equating the mass number on both sides
235 + 1 = A + 143 + 3 *1
236 = A + 143 + 3
236 = Z + 146
Z = 90
Equatoing the atomic number of both sides
92 + 0 = Z + 54 + 3*0
92 = Z + 54
Z = 92 - 54
Z = 38.