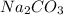Answer:
Formula of sodium carbonate is
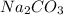 .
.
Step-by-step explanation:
Sodium carbonate is an ionic compound consists of
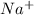 and
and
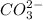 ions.
ions.
In a formula unit of sodium carbonate, there should be two
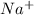 ions and one
ions and one
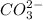 ion to maintain charge neutrality.
ion to maintain charge neutrality.
In general, formula of an ionic compound consists of
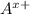 and
and
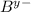 ions is written as :
ions is written as :
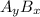 (
(
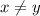 ) and AB (
) and AB (
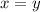 )
)
If x or y is equal to 1 then it is not written explicitly in formula.
So, formula of sodium carbonate =