Answer:
Amount of heat absorbed by water is 2604.54 J.
Step-by-step explanation:
Amount of heat absorbed by water =
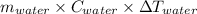
where m represents mass, C represents specific heat and
 represents change in temperature.
represents change in temperature.
Here
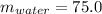 g ,
g ,
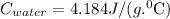 and
and
 = (final temperature - initial temperature) = (29.5-21.2)
= (final temperature - initial temperature) = (29.5-21.2)
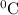 = 8.3
= 8.3
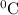
So, amount of heat heat absorbed by water
=
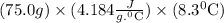
= 2604.54 J