Answer:
About 10.7 grams.
Step-by-step explanation:
The reduction reaction is represented by:
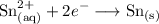
A current of 3.46 amperes (C/s) is passed through the solution for 1.40 hours. The total amount of charge is hence:
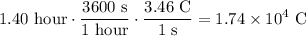
One mole of solid Sn is transferred per two moles of electrons. Hence, using Faraday's constant:
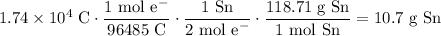
In conclusion, about 10.7 grams of tin is plated out of the solution.