Answer:
7.72 L
Step-by-step explanation:
The volume of the gas at 105 kPa would be 7.72 L.
First, let us convert the pressures into the same unit.
1 kPa = 0.00986923 atm
105 kPa = 105 x 0.00986923 = 1.0363 atm
From Boyle's law, the volume of gas is inversely proportional to the pressure of the gas. Mathematically;
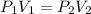
In this case,
 = 0.63,
= 0.63,
 = 12.70,
= 12.70,
 = 1.0363,
= 1.0363,
 = ?
= ?
Make
 the subject of the formula;
the subject of the formula;
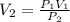
Hence,
 = 0.63 x 12.70/1.0363
= 0.63 x 12.70/1.0363
= 7.72 L