Answer:
The density of salt solution is
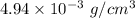 .
.
Explanation:
Given that,
Volume of certain solution, V = 7.5 litres
Mass of salt in solution, m = 37 grams
We know that,
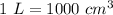
We need to find the density of salt in the solution. The density of salt is given by mass per unit volume. The formula can be written as:
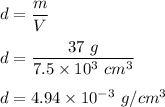
So, the density of salt solution is
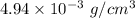 .
.