Answer : The volume of
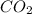 produced are, 26.7 liters.
produced are, 26.7 liters.
Explanation :
First we have to calculate the moles of
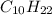
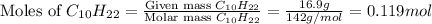
Now we have to calculate the moles of
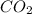 .
.
The given combustion reaction is:
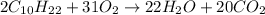
From the balanced chemical reaction we conclude that,
As, 2 moles of
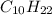 react to give 20 moles of
react to give 20 moles of
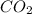
So, 0.119 moles of
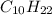 react to give
react to give
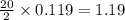 moles of
moles of
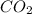
Now we have to calculate the volume of
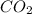 produced.
produced.
As we know that, 1 mole of gas occupies 22.4 L volume of gas.
As, 1 mole of
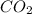 gas occupies 22.4 L volume of
gas occupies 22.4 L volume of
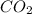 gas.
gas.
So, 1.19 mole of
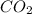 gas occupies 1.19 × 22.4 L = 26.7 L volume of
gas occupies 1.19 × 22.4 L = 26.7 L volume of
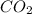 gas.
gas.
Therefore, the volume of
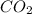 produced are, 26.7 liters.
produced are, 26.7 liters.