Answer:
Final volume is 3.50L
Step-by-step explanation:
It is possible to find volume of a gas using combined gas law:
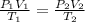
Where P is pressure, V is volume and T is temperature of 1: initial state and 2: final state
If initial state of the gas is:
1.75L of a gas is at 700K and is under 250kPa of pressure
And final state is:
298K and 53.2kPa.
Replacing:
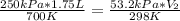
0.625L = 0.1785*V₂
3.50L = V₂
Thus, final volume is 3.50L