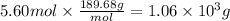Answer:
1.06 × 10³ g
Step-by-step explanation:
Let's consider the following reaction
2 TiCl₄(g) + H₂(g) ↔ 2 TiCl₃(s) + 2 HCl(g)
First, we need to calculate the moles of H₂. For that, we need to convert the pressure to atm and the temperature to Kelvin.
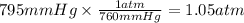
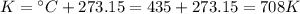
Then, we can calculate the moles of H₂ using the ideal gas equation.
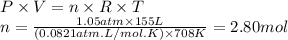
The molar ratio of TiCl₄ to H₂ is 2:1. We can use this relation to calculate the moles of TiCl₄.
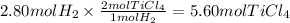
The molar mass of TiCl₄ is 189.68 g/mol. We will use this data to calculate the mass corresponding to 5.60 moles.