Answer:
The structures are attached in file.
Hydrogen bonding and intermolecular forces is the reason for ranks allotted.
Step-by-step explanation:
In determining Lewis structure, we calculate the overall number of valence electrons available for bonding. Making carbon (the least electronegative atom) the central atom in the structure, we allocate valence electrons until each atom has achieved stability.
In order of decreasing affinity to water molecules:
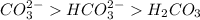
This is due to the fact that the
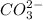 will accept protons more readily than the bicarbonate ion,
will accept protons more readily than the bicarbonate ion,
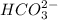 . Carbonic acid,
. Carbonic acid,
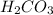 will not accept any more protons, hence it is the least attractive to water molecule, even though soluble.
will not accept any more protons, hence it is the least attractive to water molecule, even though soluble.